Inert gas saturation
Inert gas saturation is the accumulation of a gas that does not participate in metabolic processes in the body tissues. The most relevant inert gas for diving is nitrogen. Therefore we speak also about nitrogen saturation.
Contents
Basic knowledge for Open Water Diver* (OWD*)
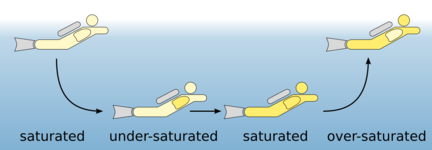
Gases can dissolve in liquids – like sugar or salt. The concentration of the gas in the liquid in the saturation state is directly related to the pressure the gas is exposed to (see Henry's law). Although nitrogen does not participate in the metabolic processes in the body – i. e. it is a so-called inert gas –, but it is present in all body tissues in a dissolved form. On the surface one is in the saturation state with reference to the body temperature and the air pressure.
With rising ambient pressure during descent the amount of breathing gas in the lungs rises respectively and so the amount of nitrogen. The nitrogen is transported via the blood into all body tissues and gets more and more saturated there. The amount of dissolved nitrogen depends on several factors:
- Ambient pressure: The deeper you dive, the more nitrogen gets saturated.
- Time: The longer you dive, the more nitrogen gets saturated.
- Circulation: The more strenuous a dive, the faster nitrogen is absorbed.
- Temperature: The colder the more nitrogen can be stored.
In normal recreational diving, most tissues are partially saturated. However, tissue with high circulation or the blood itself can also be completely saturated.
When ascending, the nitrogen which is dissolved in the body must be transported back to the lungs by the blood and released by exhalation. The pressure reduction must be so slow that nitrogen bubbles cannot form. Check the difference in opening a sparkling water bottle at once and another one very slowly. If bubbles form in our body while ascending to fast, it is called a decompression sickness.
Knowledge for Experienced Diver** (ED**)
Henry's law
Henry's law describes the solubility of gases in liquids. The amount of gas that dissolves into a liquid is directly proportional to the partial pressure of the gas and indirect proportional to the temperature of the liquid.
The partial pressure of nitrogen in our breathing gas rises with the ambient pressure. As higher the ambient pressure as more nitrogen dissolves in our body tissue. In addition, at a lower body temperature more nitrogen dissolves in the body.
Half-value time
During descent, the partial pressure of nitrogen in the breathing air increases due to the increasing ambient pressure. The body takes on more nitrogen through the lungs which is dissolved in the blood and transported into the body tissues.
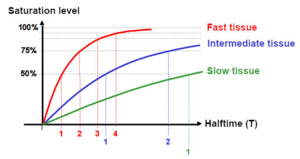
However, the saturation does not occur abruptly: At the beginning, the saturation occurs very quickly due to the large concentration difference of nitrogen. If this decreases, the saturation level increases more and more slowly.
This behavior is described by a so-called half-value time: It takes much less time for tissues to reach 50% saturation than to go from 50% saturation to fully saturated. Physiologists call the time required to reach 50% saturation in a theoretical tissue the tissue half-value time T. That means it takes the same time to go from 50% to 75% (2 T) as it did from 0% to 50% (1 T). Again it takes the them same time to go from 75% to 87.5% (3 T) as it did from 0% to 50% and so on. In theory full saturation (100%) is reached after an infinit long period of time. For pratical issues, we can say a tissue is saturated after 6 T, i. e. with a saturation level of 98,23%.
The desaturation on ascent and after the dive follows the same laws: at the beginning it is very fast and becomes slower by time. Here one assumes, analogously to the saturation, that the tissue is completely desaturated after 6 T.
Tissue groups
| tissue group | subdivision | half-value time in minutes |
|---|---|---|
| blood | fast tissues | 2,5 |
| brain | 5 | |
| spinal cord | 10 | |
| skin | middle tissues | 40 |
| muscles | 80 | |
| fatty tissue | slow tissues | 240 |
| joints | 480 |
The human body consists of different types of tissues with different properties, especially in terms of saturation behavior. For the decompression calculation, the body is divided into 6 to 16 tissue groups (so-called compartments) with different tissue half-value times. These depend on blood circulation in the tissue and nitrogen storage capacity.
The tissue groups are subdivided into slow, medium and fast tissues. Fat, for example, can store five times more nitrogen than muscle tissue, but has a relatively high tissue half-life time due to the lower blood flow and is therefore one of the slow tissues.
Tissues in which the blood flow rate may change during exercise or under the influence of temperature, such as muscles or skin, will change their tissue half-value time and, as a result, saturate faster.