Heat transfer
Between two bodies of different temperature, heat is transferred from the warmer to the colder body. Three types of heat transfer are relevant in diving: heat conduction, convection and heat transport by radiation.
Contents
Basic knowledge for Open Water Diver* (OWD*)
Heat energy is always transferred from a warm body to a colder one. The warmer body cools off, the colder one heats up. You can compare this to water: It flows always from a higher point to a deeper one.
Since water conducts heat much better than air (heat conduction and convection), you will give up body heat much faster than a shore at the same temperature. This happens also in relative warm water. In order to prevent losing to much body heat and respectively risk hypothermia, you must wear suitable thermal protection in the form of a diving suit (and corresponding supplies) when diving. This significantly reduces the heat transfer from your body to the surrounding (colder) water.
Knowledge for Experienced Diver** (ED**)
Heat is stored in the form of kinetic energy in the particles (atoms or molecules) of matter. The speed the particles depends on the ambient temperature (Brownian motion).
Heat conduction
In heat conduction, heat energy is transferred by the constant collisions particles. The measure of how well a material transfers heat is its thermal conductivity. The thermal conductivity of water is about 25 times higher than that one of air. That means, that a body loses 25 times more heat in the water than in air at the same temperature.
The loss of body heat to the water can be reduced by using insulating layers between water and body. Neoprene insulates very well because of the many embedded air bubbles. How good the insulation is depends a lot on the thickness of the material. As thicker the neoprene as less heat is passing through it and as better its insulation is.
An additional layer of gas between the body and the diving suit reduces the heat conduction even further. A dry suit is based on this principle. Ideally, it is filled with a gas with lower thermal conductivity than air (e. g. argon).
Convection
In convection, heat is lost by moving cooler air or water over a warmer body. The cold medium moves over the warm surface, heats up and dissipates heat away. You also know from everyday life: It feels much cooler on a windy day than on a day with no wind at all with the same temperature.
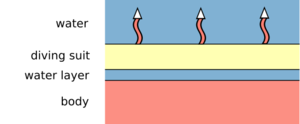
In diving, the heat transfer occurs by convection due to cooler water flowing past the diver and also by replacement of the thin water layer between the suit and the divers skin. Therefore, it is important that a diving suit sits as tight as possible, so that the exchange of water between the divers skin and the suit is as little as any possible. Semi-dry suits are equipped with additional neck, arm and leg seals and tight zippers to minimize the amount of water in the suit and convection heat dissipation even further.
Radiation
Every body emits heat in the form of thermal radiation (electromagnetic radiation in the not visible infrared spectrum) through the body's surface. The amount of heat energy released depends on the properties of the surface. For example dark surfaces emit more heat at the same temperature than lighter ones.
The same applies to the absorption of thermal radiation. A black diving suit for example heats up much more in the sun than a lighter one.
In comparison to the heat transfer by heat conduction and convection, the heat transfer via heat radiation in the water plays only a minor role.